Zebrafish as models to study Ciliopathies of the Eye and Kidney
2 Eye Hospital, Tianjin Medical University, Tianjin, 300384, China, Email: yshi@vt.edu
3 Department of Ophthalmology, The Medical University of South Carolina, Charleston, SC, 29425, USA, Email: lobo0023@umn.edu
Received: 11-Oct-2017 Accepted Date: Oct 23, 2017; Published: 30-Oct-2017
Citation: Shi Y, Su Y, Lipschutz JH, Lobo GP. Zebrafish as models to study ciliopathies of the eye and kidney. Clin Nephrol Res. October-2017;1(1):1-4.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Cilia are highly-conserved organelles projecting from the cell surface of nearly every cell type in vertebrates. Ciliary proteins have essential functions in human physiology, particularly in signaling and organ development. As cilia are a component of almost all vertebrate cells, cilia dysfunction can manifest as a constellation of features that characteristically include, retinal degeneration, renal disease and cerebral anomalies. The terminology “Ciliopathies” refers to inherited human disorders caused by genetic mutations in ciliary genes, leading to cilia dysfunctions that form an important and ever expanding multi-organ disease spectrum. Ciliopathies are a diverse class of congenital diseases, with twenty-four recognized syndromes caused by mutations in at least ninety different genes. In order to start to dissect the phenotypes of each disease associated with ciliary dysfunction it is necessary to understand the mechanisms underlying the phenotype using suitable animal models. Here, we review the advantages of the zebrafish as a vertebrate model for human ciliopathies, with a focus on ciliopathies affecting the eye and the kidney.
Keywords
Cilia, Ciliopathies, Retina, Kidney, Zebrafish
Abbreviations
AHI1: Abelson helper integration site 1 (ORF1; AHI-1; JBTS3; dJ71N10.1); ARL13B: ADP ribosylation factor like GTPase 13 (JBTS8; ARL2L1); ARL6: ADP ribosylation factor like GTPase 6 (BBS3; RP55); ARMC9: Arrowhead Regional Medical Center 9; BBS5: Bardet-Biedl syndrome 5; CC2D2A: coiled-coil and C2 domain containing 2A (MKS6; JBTS9); Cdc42: cell division cycle 42 (TKS; G25K; CDC42Hs); CEP41: centrosomal protein 41(JBTS15; TSGA14); CEP290: centrosomal protein 290 (CT87; MKS4; POC3; rd16; BBS14; JBTS5; LCA10; NPHP6; SLSN6; 3H11Ag); CSPP1: centrosome and spindle pole associated protein 1 (CSPP; JBTS21); C8ORF37: chromosome 8 open reading frame 37(RP64; BBS21; CORD16; smalltalk); Exoc5: exocyst complex component 5 (SEC10; HSEC10; SEC10P; PRO1912; SEC10L1); IFT122: intraflagellar transport 122 (CED; SPG; CED1; WDR10; WDR10p; WDR140); IFT81: intraflagellar transport81 (DV1; CDV1; CDV-1; CDV1R; CDV-1R); INPP5e: inositol polyphosphate 5-phosphatase E (CPD4; CORS1; JBTS1; MORMS; PPI5PIV; pharbin); KIAA0556: KIAA0556 (JBTS26); NBCe1: electrogenic Na+/nHCO3− cotransporter (SLC4A4); POC1B: POC1 centriolar protein B (PIX1; CORD20; TUWD12; WDR51B); PDE6D: phosphodiesterase 6D (PDED; JBTS22); RP2: RP2, ARL3 GTPase activating proteinprovided (XRP2; NME10; TBCCD2; NM23-H10; DELXp11.3); RPGR1P1: retinitis pigmentosa GTPase regulato interacting protein 1; SDCCAG8: serologically defined colon cancer antigen 8 (BBS16; CCCAP; SLSN7; NPHP10; hCCCAP; HSPC085; NY-CO-8; CCCAP SLSN7); TMEM67: transmembrane protein 67 (MKS3; JBTS6; NPHP11; TNEM67; MECKELIN); TTC26: tetratricopeptide repeat domain 26 (DYF13; IFT56; dyf-13).
Introduction
Cilia are thin rod-like microtubule-based organelles, which are found on most vertebrate cell types. Cilia can be classified as motile or non-motile (more commonly referred to as primary) cilia which arise from a common origin , the centrosome [1]. Motile cilia function mainly as motor organelles and are also found in larger organisms, including humans. For example, motile cilia are present on cells that line the trachea, where their coordinated wave-like motions carry mucus along with the inhaled dust, bacteria, and other small particles towards the mouth to be removed from the body. Primary cilia play a key role in the receptor cells of sensory systems and are responsible for cell communication [2-4]. The outer segment of the rod photoreceptor cell in the human eye is connected to its cell body with a specialized non-motile cilium. Mutations in cilia proteins have the potential to adversely affect numerous organs and tissues, and may be multifunctional [5]. Ciliopathies, referring to cilia loss and/or dysfunction in cilia development or function, cause a group of disorders associated with genetic mutations encoding defective proteins, resulting in abnormal formation or function of cilia. Clinical manifestations of ciliopathies can arise in nearly all tissue types during development and throughout life. Sensory impairments include the presence or onset of blindness, neurosensory hearing loss, altered nociception and anosmia. In addition, organ defects such as renal and liver cyst formation, airway distress, and hydrocephaly occur. Ciliopathies, phenotypes associated with cilia dysfunction, are often syndromes, such as Bardet-Biedl syndrome (BBS), Joubert syndrome (JBTS), Meckel-Gruber syndrome (MKS), Senior-Loken syndromes (SLS), Orofaciodigital syndrome (OFD), Leber’s congenital amaurosis (LCA), Ellis van Creveld syndrome, Sensenbrenner syndrome, Nephronophthisis (NPHP), Renal dysplasia, and Autosomal Polycystic kidney disease (APKD) affect multiple organs, resulting in central nervous system malformation, cystic kidney disease, polydactyly, situs inversus obesity, encephalocele and retinal dystrophy [6-8]. While disease manifestation in any organ can occur in the context of ciliopathic dysfunction, the predominant organs affected include the kidney, eye, liver and brain. Currently there is a ciliary proteome database that is an integrated community resource for the genetic and functional dissection of cilia [9]. Although ciliopathies are conveniently classified into specific syndromes, their phenotypes are best viewed as a continuum that spans a phenotypic spectrum from embryonic lethality to isolated late onset retinal degeneration [10]. Several studies support this view by demonstrating that individual ciliopathy disease genes are expressed broadly rather than discretely across the spectrum, and that mutations within the same gene can display marked phenotypic differences across and even within families [11,12]. In the ensuing text, we will provide an overview of cilia protein and ciliopathies of the kidney and eye function, highlight an ideal animal model, zebrafish, and, importantly, discuss the future direction of research into ciliopathies".
Zebrafish as models to study ciliopathies of the eye and kidney
Over the past decade zebrafish has proven to be an excellent vertebrate model for genetic analysis and imaging of cilia-related processes. The developing zebrafish larvae are largely transparent, and differentiate cilia at early stages of embryogenesis. Thus, immunostaining for ciliary proteins combined with confocal microscopy makes it easy to examine the morphology and movement of cilia during organ development in zebrafish [13-15]. Zebrafish are vertebrates, and zebrafish eyes are well-laminated structures that are functionally very similar to the eyes of other vertebrates, including humans. The eye shape of the zebrafish begins at 11.5 hours post fertilization (hpf), and the eyecup is well formed by 24 hpf. Most of the retina is subdivided into its characteristic subcellular structure by 48 hpf. The internal connecting cilia and basal body of the inner segment are observed at 50 hpf, and the outer segment is visible at 54 hpf. The first visual response can be seen around 70 hpf, and the photoreceptor cells reach an adult size of 576 hpf (24 days) [16].
Primary cilia are found in developing and mature human kidneys, which extend from the apical surface of the epithelial cells lining the nephron tubule and collecting duct. Cilia are present on endothelial cells in the developing zebrafish vasculature [14]. Zebrafish kidney vascularization and glomerular filtration occurred between 40 and 48 hpf [17]. The pronephric ducts are completely formed and patent to the exterior by 24 hours post fertilization (hpf). Cilia have been known for decades to exist, and have recently been recognized as sensory antennas that are involved in physiological functions. Nodal cilia, for example, propagate fluid flow across the embryonic node, and thereby are thought to function in the determination of left–right asymmetry. In mutations, the mis-orientation and shortening of kidney duct cilia suggest that pronephric fluid flow may be affected [15]. As a dynamic organelle, the presence, length, and composition of primary cilia are under constant regulation in order to fulfill essential functions such as signaling transduction.
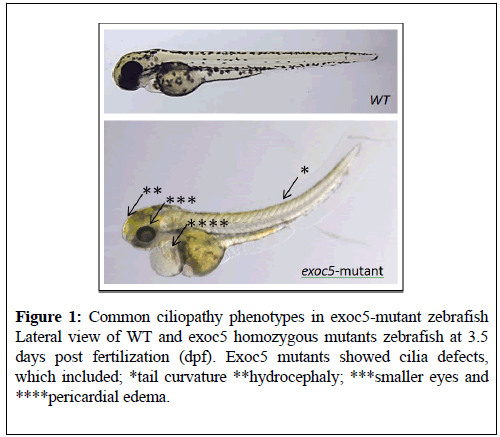
A notable feature of the zebrafish model is that cilia homozygote mutants usually manifest a curly-body axis, a phenotype that is very easy to detect during genetic screens (Figure 1) [16,18,19]. Recent advances in targeted genomic mutagenesis using TALEN and CRISPR/Cas9 nuclease systems make the zebrafish an attractive model to study reverse genetics. These approaches are valuable as tools to study the genetic bases of cilia function in a living embryo. For multiple ciliopathies, zebrafish mutants are available, including AHI1, ARL13B, ARL6, ARMC9, BBS5, CC2D2A, Cdc42, CEP41, CEP290, CSPP1, C8ORF37, Exoc5, IFT122, IFT81, INPP5e, KIAA0556, NBCe1, POC1B, PDE6D, RPGR1P1, RP2, SDCCAG8, TMEM6, TTC26, which have kidney and retina phenotypes that suggest a common mechanism underlying these defects [18,20-31] (Table 1).
| Cilia Gene modeled in Zebrafish | Eye Phenotype | Kidney Phenotype | Disease | PMID |
|---|---|---|---|---|
| AHI1 mutant | Shortened cone outer segments Cone degeneration Rhodopsin mislocalization | Kidney cysts | JBTS | 28118669 |
| ARL13B mutant | Shortened photoreceptor outer segments retinal defects | Renal cysts | JBTS | 27571019 |
| 25138100 | ||||
| 27153923 | ||||
| ARL6 mutant | Retinopathy microphthalmia | Polydactylyrenal malformations | BBS | 15314642 |
| 15258860 | ||||
| ARMC9 mutant | Retinal dystrophy | Fibrocystic kidney disease | JBTS | 28625504 |
| BBS5 Morphant | Morphants displayed retinal layering defects | Dilated cystic pronephric ducts | PKD,BBS, NPHP,MKS | 24559376 |
| 18604564 | ||||
| CC2D2A mutant | Shortened outer segments, Mislocalization of opsins and accumulation of vesicles | Pronephric cyst | JBTS, MKS | 26485645 |
| 18950740 | ||||
| Cdc42 Morphants | Smaller eyes | Cystic kidney | PKD | 23766535 |
| CEP41 Morphants | Smaller eyes | Cystic kidney | JBTS | 22246503 |
| CEP290 Morphants | Rod-cone dystrophy | Renal abnormalities | JBTS,LCA | 26301811 |
| CSPP1 Morphants | Smaller eyes | Pronephric cysts | JBTS | 24360808 |
| C8ORF37 morphants | Retinal degeneration | Renal cysts | JBTS | 27008867 |
| Exoc5 Mutants and Morphants | Smaller eyes Retinal lamination was lost Disorganization and lack of photoreceptor outer segments | Glomerular expansion left-right patterning defects | PKD | 28729419 |
| 21490950 | ||||
| IFT122 mutation | Photoreceptor degeneration | Cystic kidney | RP | 27681595 |
| IFT81 mutation | Retinal dystrophy | Kidney cyst | Nonsyndromic retinal dystrophies | 28460050 |
| INPP5e Morphants | Smaller eyes | Cystic kidney | JBTS | 27401686 |
| KIAA0556 Morphants | Oculomotor apraxia nystagmus Dysmorphic photoreceptor outer segments | Kidney cysts | JBTS | 27245168 |
| NBCe1 mutation | Smaller eyes retinal distention | Pronephric ducts defect | renal tubular acidosis, glaucoma, and cataracts | 19625604 |
| POC1B Mutation | Smaller eyes Retinal degeneration Reduce photoreceptor connecting cilia | Cystic kidney | JBTS, PKD, LCA | 25044745 |
| PDE6D Morphants | Disorganized retinal cell layers | Cloacal cysts distended pronephric tubules polydactyly and kidney hypoplasia | JBTS | 24166846 |
| RP2 Morphants | Affected the shedding of membrane discs from the distal end of the photoreceptor outer segment | Pronephric cysts | renal–retinal ciliopathies | 20729296 |
| RPGRIP1 Morphants | Smaller and underdeveloped eyes | Pronephric cyst formation | NPHP,RP | 20200501 |
| SDCCAG8 Morphants | Smaller eyes | Pronephric cysts | NPHP | 20835237 |
| TMEM67 mutation | Smaller eyes | Bilateral pronephric cysts | MKS | 23393159 |
| TTC26 Morphants | Eye morphology altered; outer segments of photoreceptor cells appeared shortened or absent | Tubule dilation; distended/dilated pronephric tubes and ducts | renal–retinal ciliopathies | 22718903 |
Table 1: Ciliopathy Genes modeled in Zebrafish and showing Eye and Kidney henotypes.
Discussion and Future Directions
Although many mechanistic aspects of ciliogenesis are now better understood, numerous questions revolving round the pathogenesis have yet to be answered. Animal models, including zebrafish in particular, will be indispensable in this regard. Cilia are well characterized in a number of organs, but the understanding of what they do varies greatly depending on the context. Photoreceptor cilia are among the best understood in terms of function and structure. In contrast to the eye, very little is known about the role of cilia in the brain, heart or the bone. The understanding of cilia function in these organs will benefit from live imaging of intact animals at developmental stages. Such imaging experiments are the strength of the zebrafish model. The zebrafish has proven to be an excellent model to study many aspects of cilia function. The ease of generating zebrafish mutants in ciliary genes using forward and reverse genetic approaches has led to a number of important findings [32-35]. Advances in imaging, such as light sheet microscopy and the use of ever more sophisticated combinations of mutant genotypes and transgenic tools to monitor cell behavior in live animals have created a fertile ground for the zebrafish model to continue generating insights into the mechanisms of ciliogenesis.
Acknowledgements
This work was supported by grants from NIH-NEI EY025034 (G.P.L), a research grant from Dialysis Clinic, Inc. (G.P.L.); Medical University of South Carolina (MUSC) start-up funds (G.P.L); VA (Merit Award I01 BX000820 to J.H.L) and NIH (P30DK074038 to J.H.L).
REFERENCES
- Anastassiia V, Alison B, Benedicte D, et al. New frontiers: discovering cilia-independent functions of cilia proteins. EMBO Reports. 2015;16:1275-87.
- Badano JL, Mitsuma N, Beales PL, et al. The ciliopathies: an emerging class of human genetic disorders. Ann Rev Genomics Hum. Genet. 2006;7:125–48.
- Bhogaraju S, Engel BD, Lorentzen E. Intraflagellar transport complex structure and cargo interactions. Cilia. 2013;2:10.
- Cardenas-Rodriguez M, Badano JL. Ciliary biology: understanding the cellular and genetic basis of human ciliopathies. Am J Med Genet C Semin Med Genet. 2009;151:263–80.
- Aoife MW, Philip LB. Ciliopathies: an expanding disease spectrum. Pediatr Nephrol. 2011;26:1039-56.
- Baker K, Beales PL. Making sense of cilia in disease: the human ciliopathies. Am J Med Genet C Semin Med Genet. 2009;151:281–95.
- Blacque OE, Leroux MR. Bardet-Biedl syndrome: an emerging pathomechanism of intracellular transport. Cell MolLife Sci. 2006;63:2145–2161.
- Smyth BJ, Snyder RW, Balkovetz DF, et al.. Recent advances in the cell biology of polycystic kidney disease. Int Rev Cytol. 2003;231:51–89.
- Adrian G, Erica ED, Nicholas K. The ciliary proteome database: an integrated community resource for genetic and functional dissection of cilia. Nature Genetics. 2006;38:961-962.
- Ware SM, Aygun MG, Hildebrandt F. Spectrum of clinical diseases caused by disorders of primary cilia. Proc Am Thorac Soc. 2011;8:444–50.
- Baala L, Audollent S, Martinovic J, et al. Pleiotropic effects of CEP290 (NPHP6) mutations extend to Meckel syndrome. Am J Hum Genet. 2007;81:170–9.
- Szymanska K, Berry I, Logan CV, et al. Founder mutations and genotypephenotype correlations in Meckel-Gruber syndrome and associated ciliopathies. Cilia. 2012;1:18.
- Shuji K, Barbara E, Irina VZ, et al. Zebrafish as a Genetic Model in Biological and Behavioral Gerontology: Where Development Meets Aging in Vertebrates – A Mini-Review. Gerontology 2009;55:430–44.
- Kallakuri S, Yu JA, Li J, et al. Endothelial Cilia Are Essential for Developmental Vascular Integrity in Zebrafish. J Am Soc Nephrol. 2015;26:864–75.
- Chengtian Zh, Jarema M. Genetic defects of pronephric cilia in zebrafish. Mech Dev. 2007;124:605-16.
- Lobo GP, Fulmer D, Guo L, et al. The exocyst is required for photoreceptor ciliogenesis and retinal development. J Biol Chem. 2017;292:14814-26.
- Drummond IA, Majumdar A, Hentschel H, et al. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development. 1998;125:4655-67.
- Lessieur EM, Fogerty J, Gaivin RJ, et al. The ciliopathy gene ahi1 is required for zebrafish cone photoreceptor outer segment morphogenesis and survival. Invest Ophthalmol Vis Sci. 2017;58:448–60.
- Sun Z, Amsterdam A, Pazour GJ, et al. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development. 2004;131:4085-93.
- Tsujikawa M, Malicki J. Genetics of photoreceptor development and function in zebrafish. Int J Dev Biol. 2004;48:925-34.
- Van De Weghe JC, Rusterholz TDS, Latour B, Grout ME, Aldinger KA, Shaheen R, et al. Mutations in ARMC9, which Encodes a Basal Body Protein, Cause Joubert Syndrome in Humans and Ciliopathy Phenotypes in Zebrafish. Am J Hum Genet. 2017;101:23-36.
- Song P, Dudinsky L, Fogerty J, et al. Arl13b Interacts With Vangl2 to Regulate Cilia and Photoreceptor Outer Segment Length in Zebrafish. Invest Ophthalmol Vis Sci. 2016;57:4517-26.
- Becker-Heck A, Zohn IE, Okabe N, et al. The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation. Nat Genet. 2011;43:79-84.
- Brand M, Heisenberg CP, Jiang YJ, et al. Mutations in zebrafish genes affecting the formation of the boundary between midbrain and hindbrain. Development. 1996;123:179-90.
- Cao Y, Park A, Sun Z. Intraflagellar transport proteins are essential for cilia formation and for planar cell polarity. J Am Soc Nephrol. 2010;21:1326-33.
- Doerre G, Malicki J. Genetic analysis of photoreceptor cell development in the zebrafish retina. Mech Dev. 2002;110:125-38.
- Drummond FJ, Sowden J, Morrison K, et al. Colon carbonic anhydrase 1: transactivation of gene expression by the homeodomain protein Cdx2. FEBS Lett. 1998;423:218-22.
- Malicki J, Neuhauss SC, Schier AF, et al. Mutations affecting development of the zebrafish retina. Development. 1996;123:263-73.
- Panizzi JR, Becker-Heck, Victoria HA, et al. Loges, CCDC103 mutations cause primary ciliary dyskinesia by disrupting assembly of ciliary dynein arms.Nat Genet. 2012;44:714–19.
- Mitchison T, Wühr M, Nguyen P, et al. Growth, interaction, and positioning of microtubule asters in extremely large vertebrate embryo cells. Cytoskeleton. 2012;69:738-50.
- Utsch B, Sayer JA, Attanasio M, et al. Identification of the first AHI1 gene mutations in nephronophthisis-associated Joubert syndrome. Pediatr Nephrol. 2006;21:32-5.
- Kishimoto N, Cao Y, Park A, Sun Z. Cystic kidney gene seahorse regulates cilia-mediated processes and Wnt pathways. Dev Cell. 2008;14:954-61.
- Omori Y, Zhao C, Saras A, et al. Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat Cell Biol. 2008;10:437-44.
- Pathak N, Austin CA, Drummond IA. Tubulin tyrosine ligase-like genes ttll3 and ttll6 maintain zebrafish cilia structure and motility. J Biol Chem. 2011;286:11685-95.
- Zhao C, Malicki J. Nephrocystins and MKS proteins interact with IFT particle and facilitate transport of selected ciliary cargos. EMBO J. 2011;30:2532–44.