An atypical case of porcine circovirus type 2 and streptococcus suis confections in pig
2 Department of Veterinary Microbiology and Infectious Diseases, Faculty of Veterinary Medicine, Vietnam National University of Agriculture, Hanoi, Viet Nam, Email: giapnv@ueh.edu.vn
3 Department of Veterinary Pathology, College of Veterinary Medicine and Research Institute for Veterinary Science, Seoul National University, Seoul 151-742, Korea, Email: KimDae@missouri.edu
4 Research Unit, BioPOA, Yongin-si, Korea, Email: parkhoonchang@gmail.com
5 Department of Veterinary Microbiology, College of Veterinary Medicine and Research Institute for Veterinary Science, Seoul National University, Seoul 151-742, Republic of Korea, Email: magic007@snu.ac.kr
#Equally contribution
Bong Kyun Park, DVM, MSc, PhD, Department of Veterinary Medicine Virology Lab, College of Veterinary Medicine and Research Institute for Veterinary Science, Seoul National University, DaeHakRo, GwanAk-Gu, Seoul 151-742, Korea, Tel: +8228801255, Fax: +8228850263, Email: parkx026@snu.ac.kr
Received: 11-Sep-2017 Accepted Date: Sep 18, 2017; Published: 25-Sep-2017
Citation: Chung HC, Nguyen VG, Kim DY, et al. An atypical case of porcine circovirus type 2 and streptococcus suis confections in pig. J Immune Disord Ther 2017;1(1):5- 8.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
BACKGROUND: Porcine circovirus diseases (PCVD) are still one of the commonest diseases of swine in Korea. The objective of the present paper is to report a case of pig co-infected with porcine circovirus type 2 and streptococcus suis.
CASE PRESENTATION: A 40 days old pig exhibited symptoms of diarrhea; a difficulty breathing and neurological sign was diagnosed for possible cause(s) of death by pathological and molecular based methods. The notable gross lesions were (i) rubbery consistency of the lungs with multiple petechial hemorrhages, (ii) congestion of lymp nodes, and brain; (iii) the wall of the small intestine was slight congestion, yellowish fluid accumulated in the lumen was observed. The main microscopic lesions were: (i) interstitial pneumonia, degeneration of lymphocyte, dense mononuclear cell infiltrated in the interstitium; (ii) germinal centers of lymph node were reduced or absent with depletion of mature lymphocytes and distribution of pyknotic nuclear material; (iii) lymphocytes, macrophages, and mainly plasma cells distributed along blood vessels within the meninges of the brain. By extensive examining previously known pathogens of nervous, respiratory, and digestive systems, only PCV2 and Streptococcus suis were systematically detected and confirmed by sequencing.
CONCLUSION: The pig described in this study suffered from an atypical porcine circovirus disease which was based on the presence of PCV2 systemically and a typical lesion of lymphoid depletion.
Keywords
Porcine Circovirus Disease, Atypical, Coinfection
Abbreviations
PCVD: Porcine Circoviruses diseases; PCV-2: Porcine Circoviruses type 2; RT-PCR: Reverse transcription polymerase chain reaction; ADV: Aujeszky’s disease virus; CSFV: Classical swine fever virus; EMCV: Encephalomyelitis virus; JEV: Japanese encephalitis virus; HPS: Hemophilus; VTEC: verotoxin- 2e producing Escherichia coli; PRRSV: Porcine reproductive and respiratory syndrome virus; SIV: Swine influenza virus; PEDV: Porcine epidemic diarrhea virus; TGEV: Transmissible gastroenteritis virus; RVA: Group A rotavirus; PDCoV: Porcine delta coronavirus.
Porcine circovirus type 2 (PCV2) is a single stranded, closed circular DNA containing 1,766-1,768 nucleotides [1]. There are four PCV2 genotypes that have been identified, of which PCV2d is a newly known genotype worldwide [2]. PCV2 is essential agent of porcine circovirus disease, including: severe systemic PCV2 infection, PCV2 lung disease, PCV2 enteric disease, PCV2 reproductive disease, and porcine dermatitis and nephropathy syndrome [3,4]. The porcine circovirus disease is also known as a multifactorial pig disease [5], of which co-infection with some virus and/or bacteria have been shown to increase replication of PCV2 and the incidence of the disease [3].
Streptococcus suis, a Gram-positive facultative anaerobe, is a major porcine pathogen that can be transmitted to human by close contact with pigs [6]. Streptococcus suis is an important pathogen in intensive swine production throughout the world and is the cause of polyserositis as well as other conditions, such as bronchopneumonia, meningitis, arthritis, pericarditis, myocarditis, endocarditis, fibrinous polyserositis, septicemia, rhinitis, and abortion [7]. Of several ubiquitous bacteria, Streptococcus suis was found in pigs ranging from non to serve PCV2 specific lesions [3].
In this study, we described on an atypical case of porcine circovirus type 2 and Streptococcus suis coinfections in a pig, which was confirmed on the basis of pathological examination and molecular based method.
Case Presentation
On June 2016, a 40 days old pig of ML farm (Junnam province, South Korea) was sent to the Department of Veterinary Medicine Virology Laboratory at Seoul National University to investigate the possible cause(s) of death. ML farm is a single-site production system (farrow-to-grower unit and a growerto- finish unit). It was described that the pig and several pigs of the same ban exhibited symptoms of diarrhea, difficulty breathing and neurological signs (ataxia, shivering). The mortality rates were estimated roundly 10%.
Laboratory Examinations
Gross and microscopic lesions
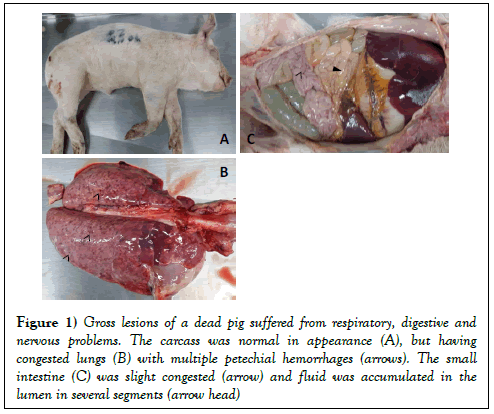
At necropsy, notable gross lesions were (i) rubbery consistency of the lungs with multiple petechial hemorrhages (Figure 1B); (ii) congestion of lymph nodes, and brain; (iii) the wall of small intestine was slight congestion, yellowish fluid accumulated in the lumen was observed (Figure 1C). Several tissues (lung, lymph nodes, and brain) were collected at 10% neutral buffered formalin for pathological diagnosis.
Figure 1: Gross lesions of a dead pig suffered from respiratory, digestive and nervous problems. The carcass was normal in appearance (A), but having congested lungs (B) with multiple petechial hemorrhages (arrows). The small intestine (C) was slight congested (arrow) and fluid was accumulated in the lumen in several segments (arrow head)
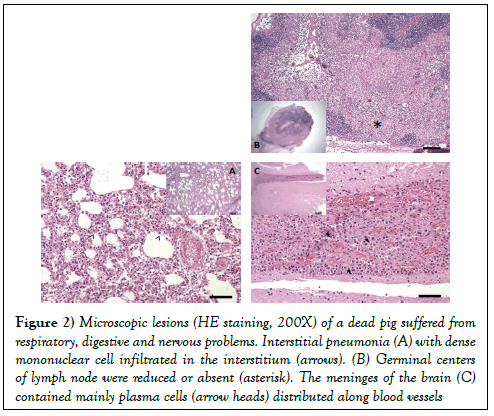
The main microscopic lesions were: (i) interstitial pneumonia, degeneration of lymphocyte, dense mononuclear cell (lymphocytes and macrophages) infiltrated in the interstitium (Figure 2A); (ii) germinal centers of lymph node were reduced or absent with depletion of mature lymphocytes and distribution of pyknotic nuclear material (Figure 2B); (iii) lymphocytes, macrophages, and mainly plasma cells distributed along blood vessels within the meninges of the brain (Figure 2C).
Figure 2: Microscopic lesions (HE staining, 200X) of a dead pig suffered from respiratory, digestive and nervous problems. Interstitial pneumonia (A) with dense mononuclear cell infiltrated in the interstitium (arrows). (B) Germinal centers of lymph node were reduced or absent (asterisk). The meninges of the brain (C) contained mainly plasma cells (arrow heads) distributed along blood vessels
Molecular based detection of common pathogens
Extensive testing was performed (on the basic of pathological changes) in order to figure out possible cause(s) of the death. Viruses and bacteria which are known to induce neurological disorder in pigs were examined, including: Aujeszky’s disease virus (ADV), Classical swine fever virus (CSFV), Encephalomyelitis virus (EMCV), Japanese encephalitis virus (JEV), Hemophilus parasuis (HPS), Streptococcus suis, and verotoxin-2e producing Escherichia coli (VTEC). Pathogens that associated with the porcine respiratory disease complex were investigated, such as: Porcine reproductive and respiratory syndrome virus (PRRSV), Swine influenza virus (SIV), Porcine circovirus type 2 (PCV2). Several major enteric pathogens of pigs were tested, such as: Porcine epidemic diarrhea virus (PEDV), Transmissible gastroenteritis virus (TGEV), Group A rotavirus (RVA), and Porcine delta corona virus (PDCoV).
Method for detection of ADV, EMCV, JEV, PRRSV, and SIV was based on commercial single or multiplex PCR (RT-PCR) kits (catalogue 3033, 3031, 3024, and 3011, Median Diagnostics, Korea). The others pathogen- specific primers were followed previous publications [8-13]. As shown in Table 1, it was striking that only 2 (Streptococcus suis and PCV2) out of 14 tested viruses/ bacteria were detected in that dead pig.
| Agents | Type of sample | ||
|---|---|---|---|
| Brain | Pooled organs | Intestine | |
| ADV | - | - | N |
| CSFV | - | - | N |
| EMCV | - | - | N |
| JEV | - | - | N |
| HPS | - | - | N |
| Strep | + | + | N |
| VTEC | Nb | N | - |
| PRRSV | - | - | N |
| SIV | - | - | N |
| PCV2 | + (T400)c | + (T401) | - |
| PEDV | N | N | - |
| TGEV | N | N | - |
| RVA | N | N | - |
| PDCoV | N | N | - |
Table 1: The results of molecular based detection of viral and bacterial pathogens from a dead pig of ML farm.
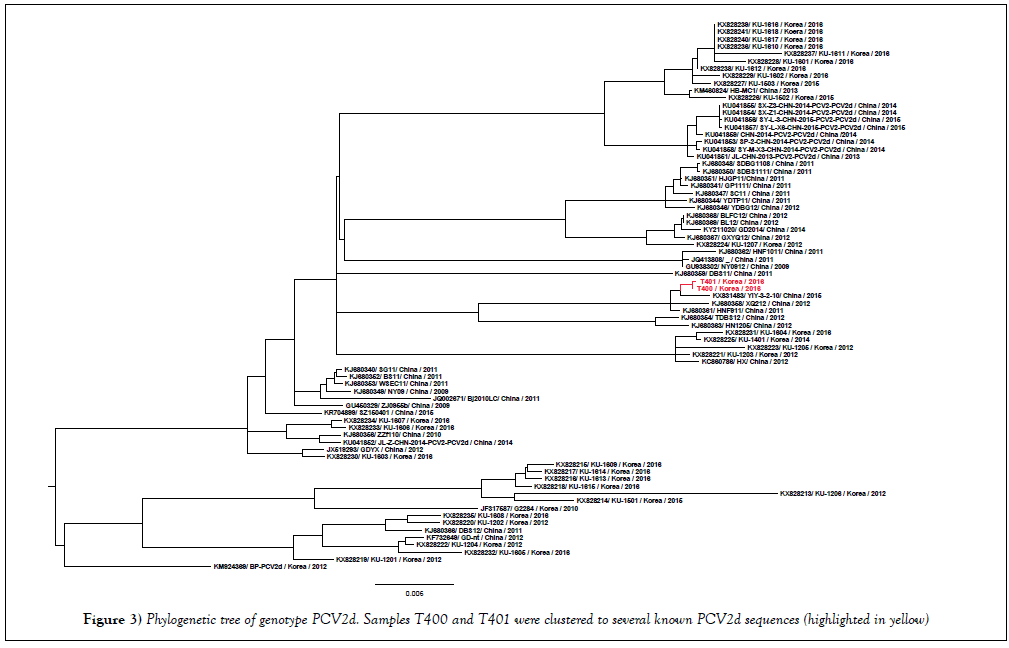
In order to confirm the specificity of the PCR reaction for Streptococcus suis, the 688 bp PCR product amplified by JP4, JP5 primers [11] was sequenced. By using BLAST tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi), the nucleotide sequence of 688 bp PCR product was 99% identical to the sequence of Streptococcus suis type 4 (GenBank CP008921). In case of PCV2, because of the recently emerging mutant of PCV2b (or PCV2d) in Korea [14], complete genome of PCV2 was obtained from positive samples (namely T400, T401). The phylogenetic analysis on the basis of the whole PCV2 genome (Figure 3) revealed that T400 and T401 (accession number KY677756, KY677757) were clustered to PCV2d genotype.
Discussion
In Korea, PRRS was very common in nearly all farms [15]. It was thus based on the gross lesions of the lung (congestion, rubbery consistency), this case was first suspected for the PRRS manifestation. However, it was failed to demonstrate the presence of PRRSV. Despite PCV2 was detected in this case, the histopathological changes of the lungs (Figure 2A) were not typical for confirmation of porcine circovirus disease (lymphohistiocytic to granulomatous interstitial Pneumonia or granulomatous bronchointerstitial pneumonia) [4]. Additionally, a few important clinical symptoms of porcine circovirus disease, such as: wasting, lymph node enlargement, etc. was observed. On the other hand, a typical lesion of the PCV2 systemic disease (lymphocyte depletion) was observed (Figure 2B).
Porcine circovirus disease is known as a multifactorial pig disease [5], of which coinfection with virus and/or bacteria have been shown to increase replication of PCV2 and the incidence of the disease [3]. In this case report, a systemic infection of Streptococcus suis was detected in brain and internal organs (Table 1). In the literature, streptococci’s in pigs were characterized by fibrinous polyserositis, fibrinous or hemorrhagic bronchopneumonia, purulent meningitis, etc. [16]. But, none of those presented in this case. It was also strange to observe mainly plasma cells along blood vessels within the meninges of the brain (Figure 2C). Nowadays, with the advantages of molecular based method, many previously unknown microorganisms which might positively related to porcine circovirus disease had been detected, such as: porcine torque teno virus [17], and porcine Circoviruses type 3 [18], etc. As the result, the presence of several novel pathogens will be further investigated for this case.
Conclusion
The pig described in this study suffered from an atypical porcine circovirus disease which was based on the presence of PCV2 systemically and a typical lesion of lymphoid depletion.
Consent
Consent was obtained from the owner of the pig for publication of this case report and any accompanying images.
Declarations
Competing Interests: The authors declare that they have no competing interests.
Availability of Data and Materials: The dataset supporting the conclusions of this article is included within the article.
Author’s Contribution
HCC, DYK, and BKP designed the study. HCC and DMG performed the experiments.CHP, NVG, DYK, DMG and HCC analyzed the data. HCC and NVG wrote the paper. BKP and KTP reviewed the literatures. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank Hye Jung Yang and Jung Ah Kim for excellent technical assistance.
Funding
The study was supported by the BioGreen 21 Program, Rural Development Administration (Grant No. PJ011184).
REFERENCES
- Hamel AL, Lin LL, Nayar GP. Nucleotide sequence of porcine circovirus associated with postweaning multisystemic wasting syndrome in pigs. J Virol 1998;72(6):5262-7.
- Xiao CT, Halbur PG, Opriessnig T. Global molecular genetic analysis of porcine circovirus type 2 (PCV2) sequences confirms the presence of four main PCV2 genotypes and reveals a rapid increase of PCV2d. The Journal of General Virology 2015;96:1830-41.
- Opriessnig T, Meng XJ, Halbur PG. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. Journal of Veterinary Diagnostic Investigation: Official Publication of the American Association of Veterinary Laboratory Diagnosticians Inc. 2007;19(6):591-615.
- Segales J. Porcine circovirus type 2 (PCV2) infections: clinical signs, pathology and laboratory diagnosis. Virus Res 2012;164(2):10-9.
- Segales J, Allan GM, Domingo M. Porcine circovirus diseases. Anim Health Res Rev 2005;6(2):119-42.
- Perch B, Kristjansen P, Skadhauge K. Group R streptococci pathogenic for man. APMIS 1968;74(1):69-76.
- MacInnes JI, Gottschalk M, Lone AG, et al. Friendship RM: Prevalence of Actinobacillus pleuropneumoniae, Actinobacillus suis, Hemophilus parasuis, Pasteurella multocida, and Streptococcus suis in representative Ontario swine herds. Canadian Journal of Veterinary Research 2008;72(3):242-8.
- Song DS, Kang BK, Oh JS, et al. Multiplex reverse transcription-PCR for rapid differential detection of porcine epidemic diarrhea virus, transmissible gastroenteritis virus, and porcine group A rotavirus. Journal of Veterinary Diagnostic Investigation: Official Publication of the American Association of Veterinary Laboratory Diagnosticians Inc. 2006;18(3):278-81.
- Lee JH, Chung HC, Nguyen VG, et al. Detection and Phylogenetic Analysis of Porcine Deltacoronavirus in Korean Swine Farms, 2015. Transbound Emerg Dis 2016;63(3):248-52.
- Yang JS, Song DS, Kim SY, et al. Detection of porcine circovirus type 2 in feces of pigs with or without enteric disease by polymerase chain reaction. Journal of Veterinary Diagnostic Investigation: Official Publication of the American Association of Veterinary Laboratory Diagnosticians Inc. 2003;15(4):369-73.
- Okwumabua O, O'Connor M, Shull E. A polymerase chain reaction (PCR) assay specific for Streptococcus suis based on the gene encoding the glutamate dehydrogenase. FEMS Microbiol Lett 2003;218(1):79-84.
- Oliveira S, Galina L, Pijoan C. Development of a PCR test to diagnose Hemophilus parasuis infections. Journal of Veterinary Diagnostic Investigation: Official Publication of the American Association of Veterinary Laboratory Diagnosticians Inc. 2001;13(6):495-501.
- Lin Z, Kurazono H, Yamasaki S, et al. Detection of Various Variant Verotoxin Genes in Escherichia coli by Polymerase Chain Reaction. Microbiology and Immunology 1993;37(7):543-8.
- Seo HW, Park C, Kang I, et al. Genetic and antigenic characterization of a newly emerging porcine circovirus type 2b mutant first isolated in cases of vaccine failure in Korea. Arch Virol 2014;159(11):3107-11.
- Lee JA, Lee NH, Lee JB, et al. Genetic diversity of the Korean field strains of porcine reproductive and respiratory syndrome virus. Infection, Genetics and Evolution: Journal of Molecular Epidemiology and Evolutionary Genetics in Infectious Diseases 2016;40:288-94.
- John VS, Wilcock B, Kierstead M. Streptococcus suis type 2 infection in swine in Ontario: a review of clinical and pathological presentations. The Canadian Veterinary Journal = La revue Veterinaire Canadienne 1982;23(3):95-7.
- Ellis JA, Allan G, Krakowka S. Effect of coinfection with genogroup 1 porcine torque teno virus on porcine circovirus type 2–associated postweaning multisystemic wasting syndrome in gnotobiotic pigs. American Journal of Veterinary Research 2008;69(12):1608-14.
- Palinski R, Pineyro P, Shang P, et al. A Novel Porcine Circovirus Distantly Related to Known Circoviruses Is Associated with Porcine Dermatitis and Nephropathy Syndrome and Reproductive Failure. J Virol 2017;91:1.